Abstract
Background: The incidence and prevalence of inhibitors among hemophilia patients in the U.S. has not been determined. The Centers for Disease Control and Prevention (CDC) has established a national inhibitor surveillance program called the Community Counts Registry for Bleeding Disorders Surveillance, through a collaboration with the American Thrombosis and Hemostasis Network and the U.S. Hemophilia Treatment Center (HTC) Network, to collect data and specimens to monitor inhibitors.
Methods: A centralized laboratory was established at the CDC to perform inhibitor tests using a protocol based on accepted methods and published findings from a pilot project, the Hemophilia Inhibitor Research Study. Specimens are collected annually and when an inhibitor is detected or suspected locally from patients with hemophilia A (HA), hemophilia B (HB), and Type 3 von Willebrand disease (VWD3) who have received treatment and are enrolled at one of 134 HTCs. In the testing protocol, a modified Nijmegen-Bethesda assay with preanalytical heat inactivation (CDC-NBA) for factor VIII (FVIII) or factor IX (FIX) is first performed. On specimens with low positive or "gray zone" results of 0.5-1.9 Nijmegen-Bethesda units (NBU) for FVIII and 0.3-0.9 NBU for FIX, chromogenic Bethesda assay (CBA) for FVIII, fluorescence immunoassay (FLI) for anti-FVIII or anti-FIX antibodies, and dilute Russell's viper venom test (DRVVT) for lupus anticoagulants are performed. Repeat specimens are requested for positive or ambiguous results in patients with negative history.
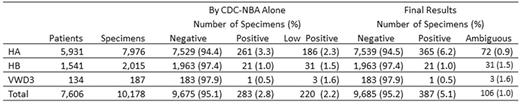
Results: Initial results on 10,178 specimens from 5,931 HA patients, 1,541 HB patients, and 134 VWD3 patients are shown in the Table. Additional testing on 141 low positive or "gray zone" FVIII specimens showed 74% to be CBA positive and 26% to be CBA negative. Of those CBA positive, 99% were also positive for IgG1 or IgG4 by FLI and were classified as true positives (104/141, 74%). Of CBA-negative specimens, 10 were also IgG1 and IgG4 negative and were classified as false positives (10/141, 7%). FLI results were positive on 26 CBA-negative specimens, which could not be classified (26/141, 18%). Final results on classified specimens are shown in the Table. Fourteen specimens were DRVVT positive, 13 of which were also CBA and FLI positive.
Follow up was conducted on two groups of specimens: Arm 1 included specimens with positive or ambiguous results at CDC from patients who were reported to have no history of inhibitor, and Arm 2 included specimens from patients with new inhibitors locally detected at the HTC within the study period. In Arm 1, of 366 positive or ambiguous FVIII specimens, 20 were from patients with negative history and 17 of 18 repeated were confirmed; and of 44 positive FIX specimens, 3 were from patients with negative history and 2 of 3 repeated were confirmed. In Arm 2, 38 locally detected positive FVIII specimens and 6 locally detected positive FIX specimens were confirmed.
Conclusions: From December 2013 through December 2016, newly positive test results on 55 FVIII and 8 FIX patients were identified and confirmed in two specimens, at least one of which was tested at the central laboratory. A cohort of 6,456 patients with negative history who were confirmed negative by central testing will be followed prospectively for inhibitor development.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal